Difference between ACTD and CTD, eCTD Regulatory affair Dossier preparation
eCTD Regulatory Overview
The electronic Common Technical Document (eCTD) is an electronic submission format for regulatory documents that is widely used in the pharmaceutical and biotech industry. The eCTD is based on the International Conference on Harmonisation (ICH) Common Technical Document (CTD) format and is accepted by regulatory authorities around the world, including the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
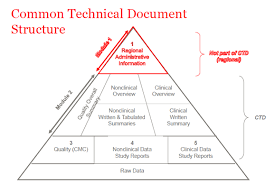
The eCTD is divided into five modules, each containing specific information about the drug product and its development:
Module 1: Administrative information, which includes documents such as the cover letter, application form, and regional specific administrative documents.
Module 2: Non-clinical study reports, which include data from animal studies, pharmacology, and toxicology studies.
Module 3: Clinical study reports, which contain data from human clinical trials, including clinical study reports, investigator’s brochures, and patient information.
Module 4: Quality information, which includes information on the manufacturing process, analytical methods, and specifications.
Module 5: Regulatory and product-specific information, which includes labeling, product monographs, and other relevant information about the product. The eCTD is designed to make the submission process for regulatory approval more efficient and streamlined. By using a standardized format, it allows for easier review and assessment of the data by regulatory authorities, which can help speed up the approval process.
The eCTD is mandatory for all new drug applications (NDAs), abbreviated new drug applications (ANDAs), and biologics license applications (BLAs) in the United States. The use of eCTD is also becoming increasingly common in other countries around the world.
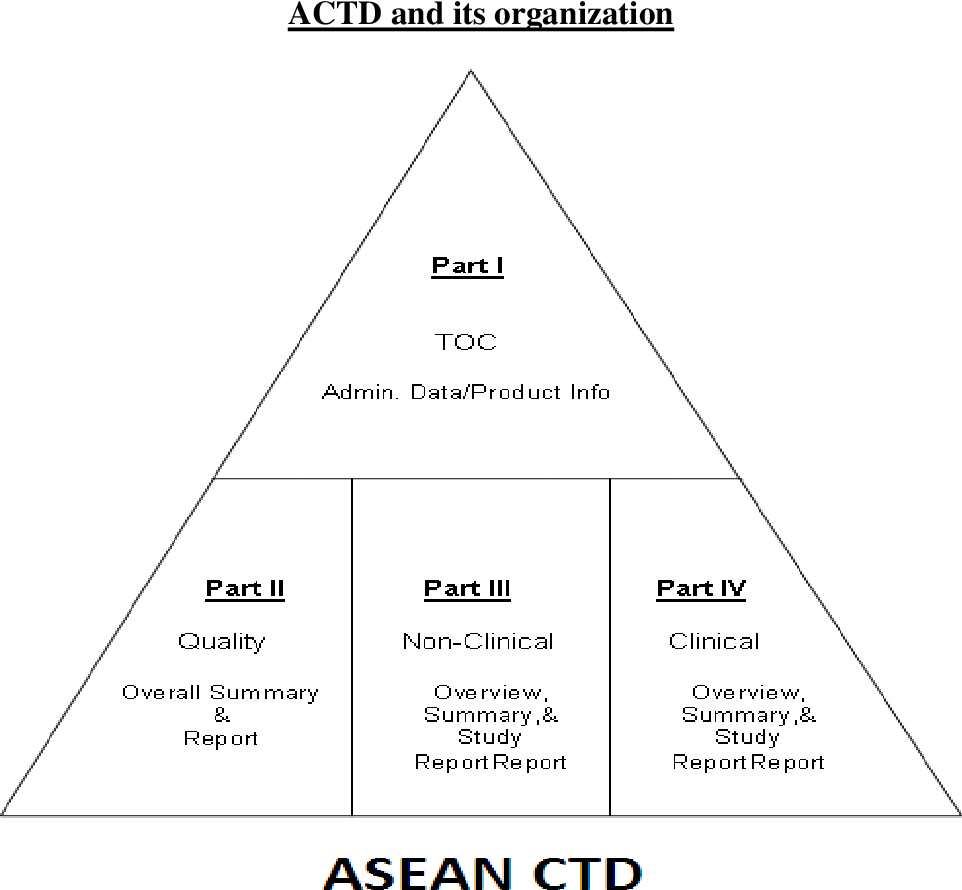
ACTD regulatory affair The ASEAN Common Technical Dossier (ACTD) is a regional format for the submission of regulatory documents in Southeast Asia. The ACTD was developed by the ASEAN Consultative Committee for Standards and Quality – Pharmaceutical Product Working Group (ACCSQ-PPWG) to harmonize the submission requirements and facilitate the registration process of pharmaceutical products in the ASEAN region. The ACTD is similar in structure to the ICH Common Technical Document (CTD), which is used in many other regions around the world. The ACTD is divided into five modules, which include:
Module 1: Administrative information, which includes documents such as the application form, product information, and a summary of the registration dossier.
Module 2: Quality information, which includes information on the quality control and manufacturing process of the product, including drug substance and drug product specifications.
Module 3: Nonclinical study reports, which include data from animal studies, pharmacology, and toxicology studies.
Module 4: Clinical study reports, which contain data from human clinical trials, including clinical study reports, investigator’s brochures, and patient information.
Module 5: Regulatory and product-specific information, which includes labeling, product monographs, and other relevant information about the product. The use of the ACTD is mandatory for the registration of pharmaceutical products in the ASEAN region. The ACTD is intended to simplify the registration process by establishing a common format for submission, which helps to reduce the regulatory burden on pharmaceutical companies and improve the efficiency of the regulatory review process.
CTD Dossier regulatory affair
eCTD module regulatory affair full information The electronic Common Technical Document (eCTD) is an electronic submission format for regulatory documents that is widely used in the pharmaceutical and biotech industry. The eCTD is based on the International Conference on Harmonisation (ICH) Common Technical Document (CTD) format and is accepted by regulatory authorities around the world, including the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
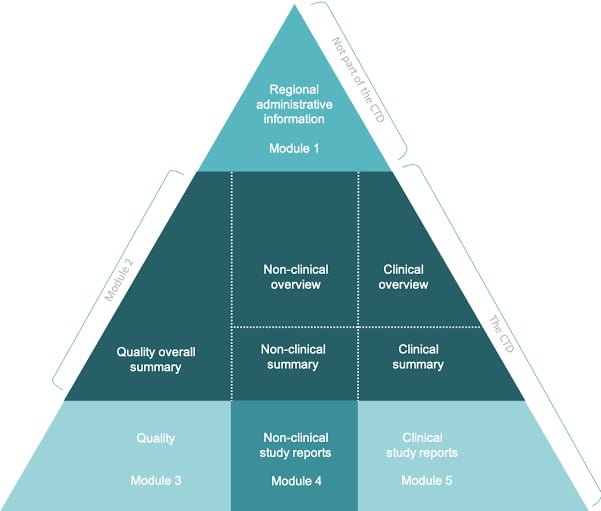
The eCTD is divided into five modules, each containing specific information about the drug product and its development:
Module 1: Administrative information, which includes documents such as the cover letter, application form, and regional specific administrative documents.
Module 2: Non-clinical study reports, which include data from animal studies, pharmacology, and toxicology studies.
Module 3: Clinical study reports, which contain data from human clinical trials, including clinical study reports, investigator’s brochures, and patient information.
Module 4: Quality information, which includes information on the manufacturing process, analytical methods, and specifications.
Module 5: Regulatory and product-specific information, which includes labeling, product monographs, and other relevant information about the product. The eCTD is designed to make the submission process for regulatory approval more efficient and streamlined. By using a standardized format, it allows for easier review and assessment of the data by regulatory authorities, which can help speed up the approval process. The eCTD is mandatory for all new drug applications (NDAs), abbreviated new drug applications (ANDAs), and biologics license applications (BLAs) in the United States. The use of eCTD is also becoming increasingly common in other countries around the world.
Overall, the eCTD is an essential component of the regulatory affairs process for pharmaceutical and biotech companies seeking approval for their drug products. ACTD regulatory affair The ASEAN Common Technical Dossier (ACTD) is a regional format for the submission of regulatory documents in Southeast Asia.
The ACTD was developed by the ASEAN Consultative Committee for Standards and Quality – Pharmaceutical Product Working Group (ACCSQ-PPWG) to harmonize the submission requirements and facilitate the registration process of pharmaceutical products in the ASEAN region. The ACTD is similar in structure to the ICH Common Technical Document (CTD), which is used in many other regions around the world. The ACTD is divided into five modules, which include:
Module 1: Administrative information, which includes documents such as the application form, product information, and a summary of the registration dossier.
Module 2: Quality information, which includes information on the quality control and manufacturing process of the product, including drug substance and drug product specifications.
Module 3: Nonclinical study reports, which include data from animal studies, pharmacology, and toxicology studies.
Module 4: Clinical study reports, which contain data from human clinical trials, including clinical study reports, investigator’s brochures, and patient information.
Module 5: Regulatory and product-specific information, which includes labeling, product monographs, and other relevant information about the product.
The use of the ACTD is mandatory for the registration of pharmaceutical products in the ASEAN region. The ACTD is intended to simplify the registration process by establishing a common format for submission, which helps to reduce the regulatory burden on pharmaceutical companies and improve the efficiency of the regulatory review process. Overall, the ACTD is an essential component of the regulatory affairs process for pharmaceutical companies seeking to register their products in the ASEAN region.
By adhering to the requirements of the ACTD, companies can help ensure a smoother and more efficient registration process, allowing them to bring their products to market more quickly and effectively.The Common Technical Document (CTD) dossier is a standard format for the submission of regulatory documents for the registration of pharmaceutical products. The CTD was developed by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) to harmonize the submission requirements for regulatory authorities around the world.
Different between ACTD and CTD
The ASEAN Common Technical Dossier (ACTD) and the Common Technical Document (CTD) are both standard formats for the submission of regulatory documents for the registration of pharmaceutical products. While there are many similarities between the two formats, there are also some key differences, which include:
Regional applicability: The ACTD is specific to the ASEAN region, while the CTD is a globally accepted format. The ACTD is used for the registration of pharmaceutical products in the ASEAN member states, while the CTD is used in many other regions around the world. Structure and content: While the basic structure of the ACTD and CTD are similar, there are some differences in the specific content and organization of the modules.
For example, the ACTD has a separate module for product information, while the CTD includes this information in Module 1. Specific requirements: There may be some specific requirements that are unique to the ACTD or CTD, depending on the regulatory authorities in each region. For example, the ACTD requires that the applicant submit a letter of authorization from the manufacturer of the drug product, while the CTD does not have this requirement.
Submission process: While the overall submission process for the ACTD and CTD is similar, there may be some differences in the specific requirements for submission, including the format of electronic submissions and the number of copies required. Overall, both the ACTD and CTD are essential components of the regulatory affairs process for pharmaceutical companies seeking to register their products. By understanding the specific requirements of each format, companies can help ensure a smoother and more efficient registration process, allowing them to bring their products to market more quickly and effectively.