Principles for Development of Biologics Similar
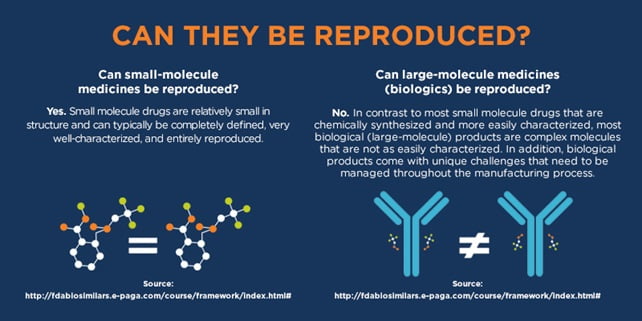
Biologics Similar biologics are created through a systematic process aimed at demonstrating their similarity to a reference biologic by conducting thorough characterization studies of molecular and quality attributes. While the preclinical and clinical evaluation of a similar biologic may be less extensive than that of the reference, it must still provide enough evidence to confirm safety, efficacy, and quality in alignment with international guidelines (WHO 2013). Typically, reduced data requirements apply only to preclinical and clinical aspects of development, while quality assessments must show comparability to the reference biologic. If any significant differences in safety, efficacy, or quality are found, more extensive evaluations will be necessary, disqualifying the product as a similar biologic. If the reference biologic is approved for multiple indications, the similar biologic can be eligible for these indications if justified, based on comparability in quality and studies, relevant literature, and whether it shares the same mechanism of action.
6.1 Selection of Reference Biologic Similar
A Reference Biologic is the original product approved after thorough evaluation, and it plays a crucial role in the development of Similar Biologics. This Reference Biologic must be utilized in all comparability assessments related to quality, preclinical, and clinical aspects. The following criteria should guide the selection of the Reference Biologic:
- The Reference Biologic must be licensed or approved in India or ICH countries and should be the innovator’s product, supported by comprehensive safety, efficacy, and quality data. As a result, another Similar Biologic cannot serve as a Reference Biologic. If the Reference Biologic is not available in India, it should at least be licensed in any ICH country. It can be imported for developing Similar Biologics for quality, preclinical, and clinical comparability.
- The same Reference Biologic must be consistently used throughout all studies that support the safety, efficacy, and quality of the Similar Biologic.
- The dosage form, strength, and route of administration for the Similar Biologic must match those of the Reference Biologic.
- The active ingredient of the Reference Biologic and the Similar Biologic must be demonstrated to be similar.


Biologics Similar
It is important to note that acceptance of an innovator product as a Reference Biologic for the evaluation of Similar Biologics does not constitute approval for its use in India.
6.2 Manufacturing Process
The manufacturer of Similar Biologics must establish a manufacturing process that produces a product with comparable identity, purity, and potency to the Reference Biologic. This process should be validated to ensure high consistency and robustness. If the host cell line for the Reference Biologic is known, using the same line for the Similar Biologic is preferred. Alternatively, any well-characterized and suitable cell line can be employed, provided there is sufficient justification to minimize significant changes in quality attributes (QAs) and to prevent the introduction of impurities that may affect clinical outcomes and immunogenicity. For establishing and characterizing cell banks, manufacturers should refer to ICH guidelines, specifically Q5A, Q5B, and Q5D.
Data Requirements for Manufacturing Process Review at Preclinical Submission
The data requirements for reviewing the manufacturing process at the preclinical submission stage must include a comprehensive overview of the entire manufacturing process, covering aspects such as the development and characterization of cell banks, clone stability, cell culture or fermentation, harvest, excipients, formulation, purification, and primary packaging interactions (if differing from the Reference Biologic). The following specific areas should be addressed:
6.2.1 Molecular Biology Considerations
Details concerning host cell cultures, including viral clearance, vectors, gene sequences, and promoters, must be provided along with appropriate diagrams. Any post-translational modifications, such as glycosylation, oxidation, deamidation, and phosphorylation, should also be explained.
6.2.2 Upstream Process Development
- A detailed description of the upstream process, including media components for cell growth, is required.
- At least three batches of reproducible fermentation data at pilot scale must be provided, ensuring the batch size is sufficient for generating preclinical data.
- The upstream process should be well-controlled and monitored.
- Kinetics data from consistency batches should include parameters like cell growth, product formation, pH, temperature, dissolved oxygen, nutrient consumption patterns, and agitation rates.
- Concentration metrics should be defined in terms of product per liter, yield, and volumetric productivity.
- Data must demonstrate that specific protein yield remains constant across upstream batches.
- Overall productivity should be shown to be reproducible and scalable.
- A detailed description of the methods for cell harvesting and protein extraction is necessary.
- Steps involved in protein purification should be outlined.
- The batch size for protein purification must also be specified.
Overview of Unit Operations in Protein Purification and Recovery
- Unit Operation Steps: Each step in the purification and recovery of proteins should be clearly described, including the quantitative yield of the product at each stage.
- Quality of Refolded Protein: When starting materials are aggregated or derived from inclusion bodies, it’s essential to assess the quality of the refolded protein. This includes:
- The refolding process details
- Specific activity measurements at various concentrations
- Dose-response curves
- Stability assessments
- Confirmation of solubility and the absence of aggregation
- Batch Recovery Consistency: The recovery consistency should be demonstrated across three consecutive purification batches, sourced from three independent cell culture or fermentation batches.
- Post-Translational Variations: Any post-translational modifications or variations should be described in detail.
- Impurity Removal: Elaborate on the methods used to eliminate impurities, including product-related variants and host cell/process-related impurities, which may pose immunogenic risks (as highlighted by EMEA guidelines from 1997).
- Virus Clearance Validation: Include studies validating the clearance of viruses during the purification process.
Clinical Trial Application Requirements
- For clinical trial submissions, adhere to the guidelines set by CDSCO. A clearly defined manufacturing process, accompanied by process controls, ensures the production of an acceptable product consistently, in line with Good Manufacturing Practices (GMP). Submission data should encompass:
- A comprehensive description of drug substance and drug product processes
- Identification of critical and key quality attributes
- Manufacturing process controls
- Critical process parameters
- Stability data
- Comparability of products manufactured at clinical scale versus reference biologics
- Data from consistency batches and/or process validation batches as applicable.
Overview of Unit Operations in Protein Purification and Recovery
- Unit Operation Steps: Each step in the purification and recovery of proteins should be clearly described, including the quantitative yield of the product at each stage.
- Quality of Refolded Protein: When starting materials are aggregated or derived from inclusion bodies, it’s essential to assess the quality of the refolded protein. This includes:
- The refolding process details
- Specific activity measurements at various concentrations
- Dose-response curves
- Stability assessments
- Confirmation of solubility and the absence of aggregation
- Batch Recovery Consistency: The recovery consistency should be demonstrated across three consecutive purification batches, sourced from three independent cell culture or fermentation batches.
- Post-Translational Variations: Any post-translational modifications or variations should be described in detail.
- Impurity Removal: Elaborate on the methods used to eliminate impurities, including product-related variants and host cell/process-related impurities, which may pose immunogenic risks (as highlighted by EMEA guidelines from 1997).
- Virus Clearance Validation: Include studies validating the clearance of viruses during the purification process.
Clinical Trial Application Requirements
For clinical trial submissions, adhere to the guidelines set by CDSCO. A clearly defined manufacturing process, accompanied by process controls, ensures the production of an acceptable product consistently, in line with Good Manufacturing Practices (GMP). Submission data should encompass:
- A comprehensive description of drug substance and drug product processes
- Identification of critical and key quality attributes
- Manufacturing process controls
- Critical process parameters
- Stability data

Biologics Similar - Comparability of products manufactured at clinical scale versus reference biologics
- Data from consistency batches and/or process validation batches as applicable.
6.3.2 Product Characterization
Characterization studies for Similar Biologics encompass physicochemical properties, biological activity, immunological characteristics, functional assays, purity (including process and product-related impurities), contamination, strength, and content. These studies should adhere to the principles outlined in the ICH Q6B guidelines and follow the Indian Pharmacopoeia Monograph where applicable.
- Structural and Physicochemical Properties: The analysis should include an assessment of both primary and higher-order structures, alongside other relevant physicochemical properties. The amino acid sequence of the Similar Biologic must be validated to match that of the Reference Biologic. Analytical methods employed should demonstrate acceptable levels of precision and accuracy, and any post-translational modifications should be identified and quantified. Significant differences must be scientifically justified and thoroughly examined in preclinical and clinical studies.
- Biological Activity: Since biological products can exhibit multiple activities, appropriate assays are necessary to characterize these activities and establish mechanisms of action and clinical effects. Data from these assays should complement the physicochemical characterization detailed in section 6.3.1. Biological assays must be validated against available international or national reference standards; if none exist, an internal reference standard should be developed per ICH guidelines.
iii. Immunological Properties: The manufacturing process for Similar Biologics can influence the level of process-related impurities and post-translational modifications, potentially affecting immunogenicity. Therefore, characterization should include comparisons with the Reference Biologic regarding specificity, affinity, binding strength, and Fc function, supplemented by animal studies.
- Purity and Impurities: Evaluating Similar Biologics requires a combination of analytical procedures to assess the following:
- Product-related variants (e.g., glycoforms, isomers)
- Product-related impurities (e.g., aggregated, oxidized, deamidated products)
- Host cell-related impurities (e.g., host cell proteins, DNA)
- Process-related impurities (e.g., residual media components, resin leachates)
Any differences in purity and impurity profiles compared to the Reference Biologic must be evaluated for their potential impact on safety and efficacy. If the Similar Biologic displays different impurities, those should be identified and characterized when feasible. Preclinical and clinical studies will help confirm that any observed impurities do not adversely affect the safety and efficacy of the Similar Biologic.
6.3.3 Specifications
Specifications for Similar Biologics, covering both drug substances and drug products, are based on quality attributes (QAs) aimed at ensuring product consistency and comparability to the Reference Biologic, in accordance with ICH Q6B guidelines. The methods used to establish these specifications may differ from those employed for product characterization and comparability assessments. Acceptance criteria should be determined based on data from the Reference Biologic and a sufficient number of batches from preclinical or clinical studies, aligning with international standards.
6.3.4 Stability
Shelf-life and storage conditions for both the drug substance and drug product should be determined through real-time stability studies. These studies must utilize containers and conditions that reflect actual storage scenarios, following relevant guidelines such as ICH Q1 A(R2), ICH Q5C, and WHO TRS 822. Conducting side-by-side accelerated and stressed stability studies comparing the Similar Biologic to the Reference Biologic will provide valuable insights into their similarity by demonstrating comparable degradation profiles.
6.4 Quality Comparability Study
A thorough quality comparison between Similar Biologics and Reference Biologics is crucial. The applicant is required to submit a complete quality dossier in line with the CDSCO guidance for industry (2008), including results from the comparability assessment before initiating clinical development. The first three consecutive standardized batches, demonstrating manufacturing consistency, should be utilized for this purpose.
Head-to-head characterization studies are necessary to compare the Similar Biologic with the Reference Biologic at the active drug product level. It is essential to confirm that the molecular structure of the active substance in the Similar Biologic aligns with that of the Reference Biologic. However, if the required analyses of quality attributes for the Reference Biologic can be performed at the finished product stage, testing of the isolated active ingredient may not be necessary. Any differences identified between the Similar Biologic and the Reference Biologic should be evaluated for their potential impact on safety and efficacy, and further characterization studies may be warranted.
Minor differences between a Similar Biologic and a Reference Biologic may occur; however, sufficient data must be provided to demonstrate that these differences do not affect safety and efficacy. The quality comparison should focus on Quality Attributes (QAs) using advanced high-resolution analytical techniques sensitive enough to detect product changes. These QAs can be categorized into Critical Quality Attributes (CQA) and Key Quality Attributes (KQA):
- Critical Quality Attributes (CQA): These attributes directly impact clinical safety or efficacy and include factors affecting the known mechanisms of action. CQAs must be controlled within limits established based on the Reference Biologic.
- Key Quality Attributes (KQA): While these attributes do not directly influence clinical safety or efficacy, they are important for product and process consistency. KQAs must be controlled within acceptable limits, allowing for slight variations compared to the Reference Biologic.
A list of routine analytical tests for a comprehensive comparability assessment of Critical and Key Quality Attributes is provided in Annexure-II. This serves as guidance, proposing a framework to establish analytical similarity that considers molecular structure, function, and heterogeneity, though specific evaluations must be tailored for each biologic molecule.