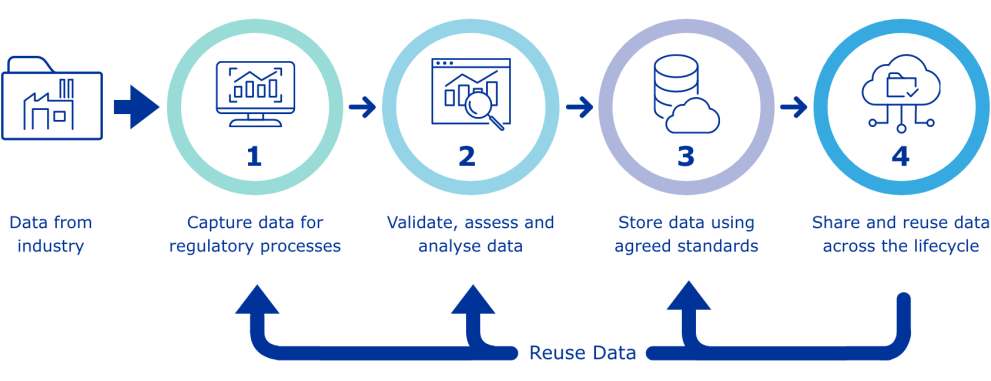
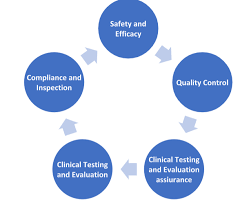
New Drug Discovery and developments-Pharmaceutical regulatory affairs-2024
New Drug Discovery and developments-Pharmaceutical regulatory affairs-2024 Background-New Drug Discovery and developments Drug discovery is a complex and multi-step process that involves identifying chemical compounds …