EMA Guidelines: Quality and Similarity of Topically Applied, Locally Acting Skin Products
Skin Products: The European Medicines Agency (EMA) has recently revised its guideline on “Quality and Equivalence of Topically Applied, Locally Acting Skin Products.” This updated document offers detailed standards regarding the quality and equivalence of skin products applied directly to the surface. It is crucial for ensuring that these products adhere to uniform safety, efficacy, and quality standards, particularly when evaluating generic or similar products against the original reference products.
This guideline focuses on locally applied, locally acting medications intended for cutaneous use, but it can also be relevant for other types of products, such as those for auricular or ocular applications, as well as locally acting vaginal treatments. It offers specific guidance on:
Skin Products
- The quality standards for cutaneous products that are not addressed by existing guidelines or applicable Pharmacopoeia standards.
- Equivalence testing for cutaneous products, emphasizing the importance of this testing over traditional therapeutic equivalence studies that involve clinical endpoints.
The guideline offers insights on various models and studies that can be utilized to assess equivalence in terms of:
- Quality
- Efficacy
- Safety
These elements, when combined, support a claim of therapeutic equivalence, particularly when the method of administration remains consistent.
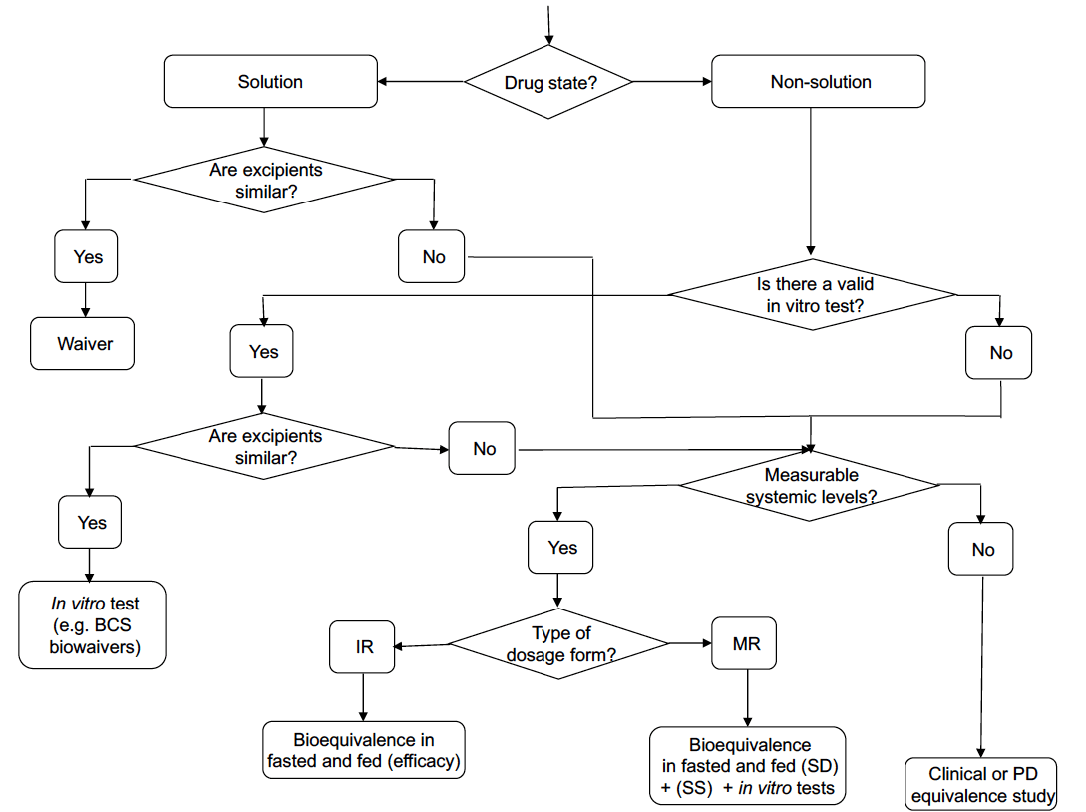
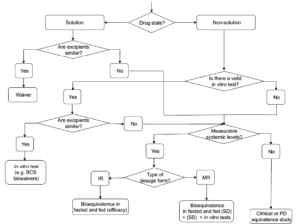
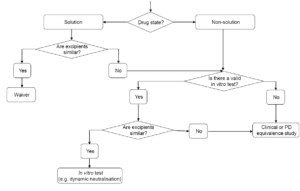
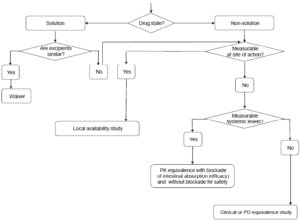
Existing guidelines highlight that modifications in formulation, dosage form, administration method, or manufacturing processes can significantly affect the efficacy and safety of topical products. While therapeutic equivalence studies with clinical endpoints are typically essential, this guideline suggests that alternative models may also be developed or employed. It includes detailed information on using in vitro and in vivo models in a systematic approach to establish therapeutic equivalence.
Current guidelines indicate that modifications to formulation, dosage form, administration method, or manufacturing processes can significantly influence the efficacy and safety of topical products. While studies demonstrating therapeutic equivalence with clinical endpoints are generally required, alternative models can also be utilized or created. This guideline elaborates on the application of in vitro and in vivo models in a systematic approach to establish therapeutic equivalence.


Additionally, the guideline underscores the importance of thorough pharmaceutical development, which includes ensuring the formulation and manufacturing processes are appropriate. Key factors such as particle size, viscosity, and spreadability can impact product performance on the skin, and these aspects must be carefully controlled and justified throughout the development process.
The guideline pertains to locally applied and locally acting medicinal products for cutaneous use (referred to as cutaneous products), but it may also be applicable to other medications, such as those for auricular or ocular use and locally acting vaginal products. Specific guidance includes:
- Quality standards for cutaneous products not addressed by other guidelines or relevant Pharmacopoeia standards.
- Equivalence testing for cutaneous products instead of relying solely on therapeutic equivalence studies with clinical endpoints.
Current guidelines indicate that changes in formulation, dosage form, method of administration, or manufacturing processes can significantly impact the efficacy and safety of cutaneous products. While therapeutic equivalence studies with clinical endpoints are generally necessary, alternative models can also be utilized or developed.
This guidance is intended to assist in developing and justifying product-specific equivalence protocols. Additionally, the annexes provide design guidance for:




- In vitro release studies
- In vitro human skin permeation assessments
- In vivo stratum corneum sampling (tape stripping)
- In vivo vasoconstriction assays for corticosteroids
The quality guidelines is relevant for new marketing authorization applications as well as post-approval changes, while the equivalence guidance applies to specific cases demonstrating the therapeutic equivalence of a new cutaneous medicinal product in comparison to an existing one.
The variety of cutaneous products is extensive due to the complex nature of the skin, the diverse conditions they aim to treat, and the different needs of patients. As a result, the guideline cannot provide a one-size-fits-all procedure; instead, it offers general recommendations tailored to the characteristics of specific medicinal products. This guidance builds upon existing regulatory frameworks and reflects current scientific knowledge.
It includes quality standards for cutaneous products that are not addressed by other general quality guidelines or relevant Pharmacopoeial standards. A clear understanding of the product’s indication, target population, and site of action is essential for making informed decisions regarding pharmaceutical form, composition, and method of administration.
The primary functions of the drug product must be well understood. In many cases, the goal is simply to deliver the active substance to the skin’s surface. However, bioavailability can often be enhanced by incorporating excipients that alter the thermodynamic activity of the active substance, such as solubilizers and supersaturants, or by using penetration enhancers that modify diffusion or disrupt the skin barrier. Factors like occlusion and the choice of vehicle—such as moisturizers, emollients, and excipients that evaporate post-application—can also significantly impact the condition being treated.
The formulation should be developed based on solid prior knowledge, a well-established scientific rationale, and empirical evidence. The quality characteristics should be assessed using multiple batches that are representative of the marketed product.
Current guidelines indicate that for topical products, changes in formulation, dosage form, method of administration, or manufacturing processes can significantly affect efficacy and safety. While therapeutic equivalence studies with clinical endpoints are generally necessary, alternative models may also be used or developed.
This guideline provides detailed information on how in vitro and in vivo models can substitute for clinical data in establishing therapeutic equivalence, following a stepwise approach.
SCOPE:Skin Products
The guideline is applicable to locally applied and locally acting medicinal products intended for cutaneous use. These principles may also extend to other topical medications, such as those for auricular or ocular applications, as well as locally acting products for vaginal mucosa or nails.
It provides guidance on the quality of cutaneous products containing chemical active substances that are not addressed by existing general quality guidelines. Additionally, it outlines equivalence testing for cutaneous products to support claims of therapeutic equivalence with reference medicinal products, serving as an alternative to therapeutic equivalence studies with clinical endpoints.
The quality guidance (Section 4) is relevant for new marketing authorization applications and post-approval changes. The equivalence guidance (Section 5) applies to specific cases where a new medicinal product must demonstrate therapeutic equivalence to an existing one. This section is also applicable to post-approval changes when a risk assessment indicates a potential impact on quality, safety, or efficacy.
Furthermore, for applications relying on literature to establish the safety and efficacy of a medicinal product, the relevance of that literature must be supported by equivalence bridging data related to the product described.

However, the equivalence guidance (Section 5) does not apply in the following situations:
- To biological medicinal products (refer to guidelines on similar biological medicinal products).
- To herbal medicinal products.
- When equivalence in efficacy is established through therapeutic equivalence clinical trials.
- When the pharmaceutical forms of the test and reference products differ.