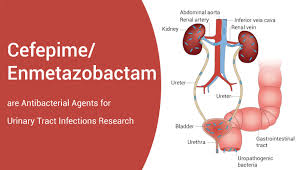
Cefepime Orchid Pharma has been granted by the (DCGI) for its Cefepime and Enmetazobactam antibiotic 2024.
Cefepime Orchid Pharma has been granted approval by the Drug Controller General of India (DCGI) for its Cefepime and Enmetazobactam antibiotic. Cefepime Orchid Pharma, headquartered …