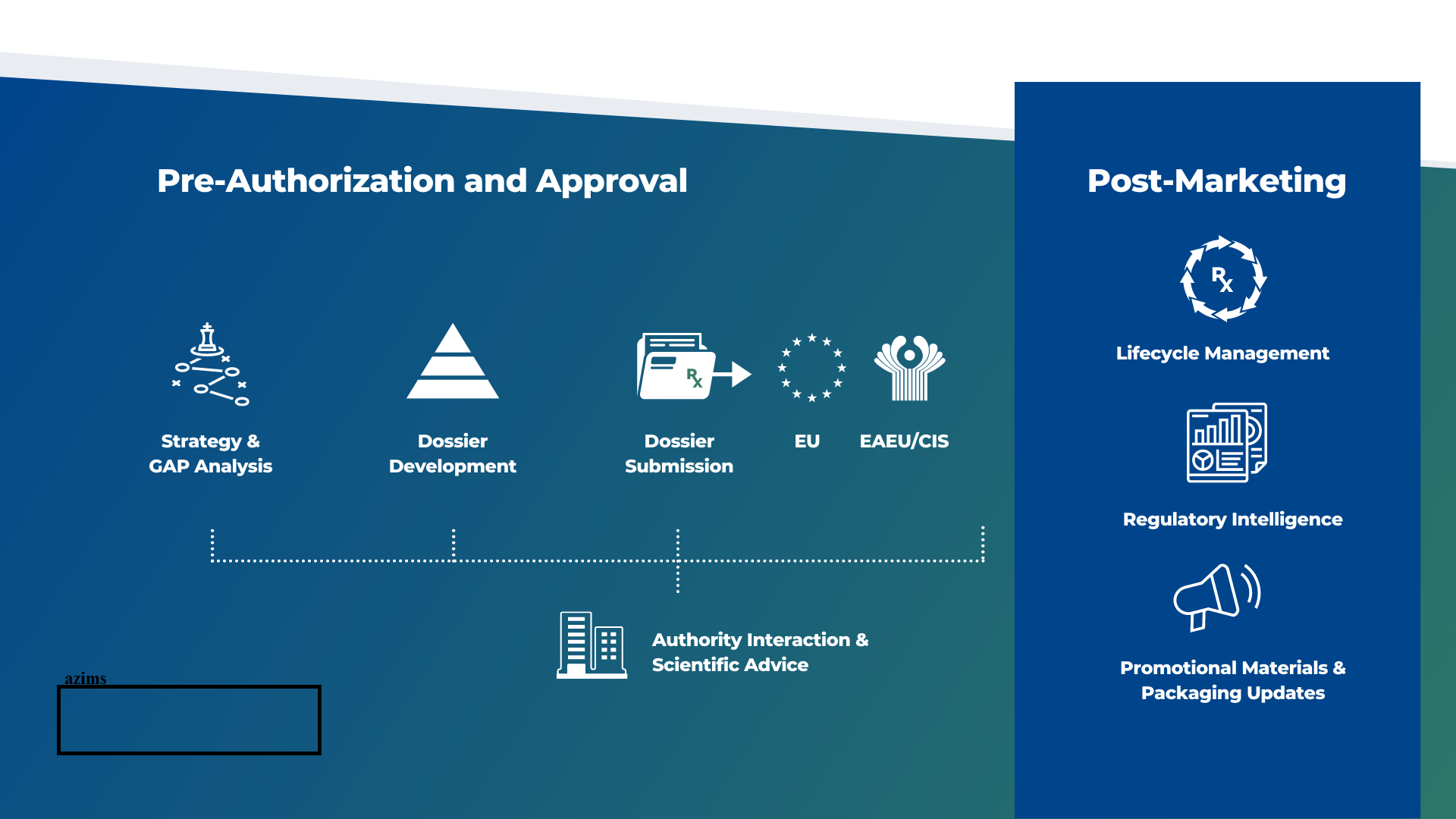
Regulatory Affairs Requirement for submission dossier
Regulatory Affairs Requirement: USFDA
Regulatory Affairs Requirement The United States Food and Drug Administration (FDA) is responsible for ensuring the safety and effectiveness of a wide range of products, including drugs, medical devices, food, and cosmetics. In order to comply with FDA requirements, regulatory affairs professionals must have a deep understanding of the regulatory framework in which their organization operates and must be able to navigate the complex requirements of the FDA.
The specific requirements for compliance with FDA regulations will vary depending on the type of product or service being offered. However, in general, regulatory affairs professionals must ensure that their organization is in compliance with applicable regulations, such as the Federal Food, Drug, and Cosmetic Act (FD&C Act), the Code of Federal Regulations (CFR), and other guidance documents and standards.
In the context of drug development, for example, regulatory affairs professionals must ensure that their organization obtains appropriate approvals and authorizations from the FDA, such as IND applications and New Drug Applications (NDAs). They must also ensure that all clinical trials and studies are conducted in compliance with Good Clinical Practice (GCP) and other applicable regulations and guidelines, and that all data is collected and managed in compliance with FDA requirements.
For medical devices, regulatory affairs professionals must ensure that their organization obtains appropriate premarket approvals or clearances from the FDA, such as Premarket Approval (PMA) or 510(k) clearance. They must also ensure that all devices are designed and manufactured in compliance with applicable regulations, such as the Quality System Regulation (QSR).
In addition, regulatory affairs professionals must ensure that all labeling and promotional materials are in compliance with FDA regulations and guidelines, and that adverse events and other safety concerns are reported to the FDA in a timely manner. They may also be involved in responding to inquiries and inspections from the FDA, providing information and evidence as required.
Overall, compliance with FDA regulations is essential for the safety and effectiveness of products and services offered in the United States. Regulatory affairs professionals play a critical role in ensuring that their organization is in compliance with these regulations, working closely with the FDA and other stakeholders to ensure that all aspects of the organization’s operations meet regulatory requirements.
Regulatory Affairs Requirement: EMA
In Europe, the regulatory framework for medical products, including drugs and medical devices, is governed by the European Medicines Agency (EMA) and the European Commission. The specific requirements for compliance with European Union (EU) regulations will vary depending on the type of product or service being offered, but in general, regulatory affairs professionals must ensure that their organization is in compliance with applicable regulations, such as the EU Directives and Regulations.
In the context of drug development, for example, regulatory affairs professionals must ensure that their organization obtains appropriate approvals and authorizations from the EMA, such as the centralized or decentralized procedure for Marketing Authorization Applications (MAAs). They must also ensure that all clinical trials and studies are conducted in compliance with Good Clinical Practice (GCP) and other applicable regulations and guidelines, and that all data is collected and managed in compliance with European requirements.
For medical devices, regulatory affairs professionals must ensure that their organization obtains appropriate CE marking for their products, demonstrating compliance with the European Medical Devices Regulation (MDR) or In Vitro Diagnostic Medical Devices Regulation (IVDR). They must also ensure that all devices are designed and manufactured in compliance with applicable regulations, such as the Quality Management System (QMS) requirements.
In addition, regulatory affairs professionals must ensure that all labeling and promotional materials are in compliance with EU regulations and guidelines, and that adverse events and other safety concerns are reported to the relevant regulatory authorities in a timely manner. They may also be involved in responding to inquiries and inspections from the regulatory authorities, providing information and evidence as required.
Overall, compliance with EU regulations is essential for the safety and effectiveness of medical products offered in the European market. Regulatory affairs professionals play a critical role in ensuring that their organization is in compliance with these regulations, working closely with the EMA, European Commission and other stakeholders to ensure that all aspects of the organization’s operations meet regulatory requirements.
Regulatory Affairs Requirement: DCGI
The Drug Controller General of India (DCGI) is responsible for regulating drugs and medical devices in India. Regulatory affairs professionals operating in India must have a deep understanding of the regulatory framework in which their organization operates and must be able to navigate the complex requirements of the DCGI.
In order to comply with DCGI requirements, regulatory affairs professionals must ensure that their organization is in compliance with applicable regulations, such as the Drugs and Cosmetics Act and Rules. These regulations govern the import, manufacture, distribution, and sale of drugs and medical devices in India.
In the context of drug development, regulatory affairs professionals must ensure that their organization obtains appropriate approvals and authorizations from the DCGI, such as the approval of clinical trial applications and the grant of marketing authorizations. They must also ensure that all clinical trials and studies are conducted in compliance with Good Clinical Practice (GCP) and other applicable regulations and guidelines, and that all data is collected and managed in compliance with DCGI requirements.
For medical devices, regulatory affairs professionals must ensure that their organization obtains appropriate licenses and approvals from the DCGI, such as the Import License or the Manufacturing License. They must also ensure that all devices are designed and manufactured in compliance with applicable regulations, such as the Medical Device Rules.
In addition, regulatory affairs professionals must ensure that all labeling and promotional materials are in compliance with DCGI regulations and guidelines, and that adverse events and other safety concerns are reported to the DCGI in a timely manner. They may also be involved in responding to inquiries and inspections from the DCGI, providing information and evidence as required.
Overall, compliance with DCGI regulations is essential for the safety and effectiveness of drugs and medical devices offered in India. Regulatory affairs professionals play a critical role in ensuring that their organization is in compliance with these regulations, working closely with the DCGI and other stakeholders to ensure that all aspects of the organization’s operations meet regulatory requirements.
Regulatory Affairs Requirement: TGA
The Therapeutic Goods Administration (TGA) is the regulatory agency responsible for regulating therapeutic goods in Australia. Regulatory affairs professionals operating in Australia must have a deep understanding of the regulatory framework in which their organization operates and must be able to navigate the complex requirements of the TGA.
In order to comply with TGA requirements, regulatory affairs professionals must ensure that their organization is in compliance with applicable regulations, such as the Therapeutic Goods Act and Regulations. These regulations govern the import, manufacture, distribution, and sale of therapeutic goods in Australia.
In the context of drug development, regulatory affairs professionals must ensure that their organization obtains appropriate approvals and authorizations from the TGA, such as the approval of clinical trial applications and the grant of marketing authorizations. They must also ensure that all clinical trials and studies are conducted in compliance with Good Clinical Practice (GCP) and other applicable regulations and guidelines, and that all data is collected and managed in compliance with TGA requirements.
For medical devices, regulatory affairs professionals must ensure that their organization obtains appropriate conformity assessments and approvals from the TGA, such as the Australian Register of Therapeutic Goods (ARTG) listing. They must also ensure that all devices are designed and manufactured in compliance with applicable regulations, such as the Australian Regulatory Guidelines for Medical Devices (ARGMD).
In addition, regulatory affairs professionals must ensure that all labeling and promotional materials are in compliance with TGA regulations and guidelines, and that adverse events and other safety concerns are reported to the TGA in a timely manner. They may also be involved in responding to inquiries and inspections from the TGA, providing information and evidence as required.
Overall, compliance with TGA regulations is essential for the safety and effectiveness of therapeutic goods offered in Australia. Regulatory affairs professionals play a critical role in ensuring that their organization is in compliance with these regulations, working closely with the TGA and other stakeholders to ensure that all aspects of the organization’s operations meet regulatory requirements.
Regulatory Affairs Requirement: ROW
Regulatory affairs requirements for drugs and medical devices in the Rest of the World (ROW) can vary widely depending on the country or region in question. However, there are some general principles and best practices that regulatory affairs professionals operating in the ROW can follow.
In general, regulatory affairs professionals must have a deep understanding of the regulatory framework in which their organization operates in the ROW, and must be able to navigate the complex requirements of each country or region. They must ensure that their organization is in compliance with applicable regulations, guidelines, and standards in each country or region where their products are being developed, manufactured, or sold.
For drug development, regulatory affairs professionals must ensure that their organization obtains appropriate approvals and authorizations from the regulatory agency or agencies in each country or region. This may include approval of clinical trial applications, marketing authorizations, and other regulatory approvals. They must also ensure that all clinical trials and studies are conducted in compliance with Good Clinical Practice (GCP) and other applicable regulations and guidelines, and that all data is collected and managed in compliance with local regulatory requirements.
For medical devices, regulatory affairs professionals must ensure that their organization obtains appropriate conformity assessments and approvals from the regulatory agency or agencies in each country or region. This may include registration or listing on a medical device registry or database, obtaining certification from a notified body, and compliance with local regulations and guidelines.
In addition, regulatory affairs professionals must ensure that all labeling and promotional materials are in compliance with local regulatory requirements and guidelines, and that adverse events and other safety concerns are reported to the relevant regulatory agency in each country or region in a timely manner. They may also be involved in responding to inquiries and inspections from regulatory agencies, providing information and evidence as required.
Overall, compliance with regulatory requirements in the ROW is essential for the safety and effectiveness of drugs and medical devices offered in those countries or regions. Regulatory affairs professionals play a critical role in ensuring that their organization is in compliance with these regulations, working closely with regulatory agencies and other stakeholders to ensure that all aspects of the organization’s operations meet regulatory requirements.
Regulatory Affairs Requirement: ANVISA
ANVISA (Agência Nacional de Vigilância Sanitária) is the regulatory agency responsible for regulating health products in Brazil. Regulatory affairs professionals operating in Brazil must have a deep understanding of the regulatory framework in which their organization operates and must be able to navigate the complex requirements of ANVISA.
In order to comply with ANVISA requirements, regulatory affairs professionals must ensure that their organization is in compliance with applicable regulations, such as the Brazilian Health Surveillance Agency Law (Lei nº 9.782/1999) and the regulations and guidelines issued by ANVISA.
For drug development, regulatory affairs professionals must ensure that their organization obtains appropriate approvals and authorizations from ANVISA, such as the approval of clinical trial applications and the grant of marketing authorizations. They must also ensure that all clinical trials and studies are conducted in compliance with Good Clinical Practice (GCP) and other applicable regulations and guidelines, and that all data is collected and managed in compliance with ANVISA requirements.
For medical devices, regulatory affairs professionals must ensure that their organization obtains appropriate conformity assessments and approvals from ANVISA, such as registration or notification of the device. They must also ensure that all devices are designed and manufactured in compliance with applicable regulations, such as the Brazilian regulations for medical devices (RDC nº 185/2001).
In addition, regulatory affairs professionals must ensure that all labeling and promotional materials are in compliance with ANVISA regulations and guidelines, and that adverse events and other safety concerns are reported to ANVISA in a timely manner. They may also be involved in responding to inquiries and inspections from ANVISA, providing information and evidence as required.
Overall, compliance with ANVISA regulations is essential for the safety and effectiveness of health products offered in Brazil. Regulatory affairs professionals play a critical role in ensuring that their organization is in compliance with these regulations, working closely with ANVISA and other stakeholders to ensure that all aspects of the organization’s operations meet regulatory requirements.
Regulatory Affairs Requirement: HEALTH CANADA
Health Canada is the regulatory agency responsible for regulating drugs, medical devices, and other health products in Canada. Regulatory affairs professionals operating in Canada must have a deep understanding of the regulatory framework in which their organization operates and must be able to navigate the complex requirements of Health Canada.
In order to comply with Health Canada requirements, regulatory affairs professionals must ensure that their organization is in compliance with applicable regulations, such as the Food and Drugs Act and the Medical Devices Regulations.
For drug development, regulatory affairs professionals must ensure that their organization obtains appropriate approvals and authorizations from Health Canada, such as the approval of clinical trial applications and the grant of marketing authorizations. They must also ensure that all clinical trials and studies are conducted in compliance with Good Clinical Practice (GCP) and other applicable regulations and guidelines, and that all data is collected and managed in compliance with Health Canada requirements.
For medical devices, regulatory affairs professionals must ensure that their organization obtains appropriate licenses and approvals from Health Canada, such as a medical device license or an authorization to import or sell a medical device in Canada. They must also ensure that all devices are designed and manufactured in compliance with applicable regulations, such as the Canadian Medical Devices Regulations.
In addition, regulatory affairs professionals must ensure that all labeling and promotional materials are in compliance with Health Canada regulations and guidelines, and that adverse events and other safety concerns are reported to Health Canada in a timely manner. They may also be involved in responding to inquiries and inspections from Health Canada, providing information and evidence as required.
Overall, compliance with Health Canada regulations is essential for the safety and effectiveness of health products offered in Canada. Regulatory affairs professionals play a critical role in ensuring that their organization is in compliance with these regulations, working closely with Health Canada and other stakeholders to ensure that all aspects of the organization’s operations meet regulatory requirements.

WHO Requirements:
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. While WHO does not have regulatory authority over health products, it plays an important role in establishing international norms and standards for the development, regulation, and use of health products. Regulatory affairs professionals operating in WHO Member States must have a deep understanding of the regulatory framework in which their organization operates and must be able to navigate the complex requirements of WHO.
In order to comply with WHO requirements, regulatory affairs professionals must ensure that their organization is in compliance with applicable regulations, such as the International Health Regulations and the guidelines issued by WHO.
For drug development, regulatory affairs professionals must ensure that their organization follows the principles of Good Manufacturing Practice (GMP), Good Laboratory Practice (GLP), and Good Clinical Practice (GCP) as outlined in WHO guidelines. They must also ensure that all clinical trials and studies are conducted in compliance with GCP and other applicable regulations and guidelines, and that all data is collected and managed in compliance with WHO requirements.
WHO plays an important role in prequalifying medicines for use in international procurement. Prequalification involves a thorough assessment of the quality, safety, and efficacy of a medicine, as well as an assessment of the manufacturing facility and the quality control systems in place. Regulatory affairs professionals may be involved in the prequalification process, ensuring that the medicines produced by their organization meet WHO standards and are eligible for procurement by UN agencies and other organizations.
For medical devices, regulatory affairs professionals must ensure that their organization follows the principles of Good Manufacturing Practice (GMP) and Good Distribution Practice (GDP) as outlined in WHO guidelines. They must also ensure that all devices are designed and manufactured in compliance with applicable regulations, such as the International Medical Device Regulators Forum (IMDRF) guidance documents.
WHO also plays an important role in establishing norms and standards for the use of health products. For example, WHO develops guidelines for the use of specific medicines in certain disease areas and provides guidance on the use of essential medicines. Regulatory affairs professionals may be involved in the development of these guidelines and in ensuring that the use of health products in their organization is in compliance with WHO standards.
In addition, regulatory affairs professionals must ensure that all labeling and promotional materials are in compliance with WHO regulations and guidelines, and that adverse events and other safety concerns are reported to WHO in a timely manner. They may also be involved in responding to inquiries and inspections from WHO, providing information and evidence as required.
Overall, compliance with WHO regulations and guidelines is essential for the safety and effectiveness of health products offered internationally. Regulatory affairs professionals play a critical role in ensuring that their organization is in compliance with these regulations, working closely with WHO and other stakeholders to ensure that all aspects of the organization’s operations meet regulatory requirements.