What is in-vitro-in vivo correlation (IVIVC)
In-vitro-in vivo correlation (IVIVC) is a mathematical model that predicts the connection between an in vitro characteristic of a drug formulation and its corresponding in vivo effect. In the context of formulation development, the in vitro characteristic typically refers to dissolution or drug release, while the in vivo effect relates to the drug’s plasma concentration or the rate and extent of absorption. Essentially, IVIVC illustrates how drug release in a dissolution test correlates with the amount of drug that enters the bloodstream after administration. This correlation is particularly relevant when a drug exhibits high solubility, and the dissolution process is the limiting factor for absorption. IVIVC plays a crucial role in various scenarios, especially for extended-release oral formulations.
Why Conduct IVIVC correlation?
Regulatory authorities recommend the use of in vitro-in vivo correlation (IVIVC) models for most modified-release dosage forms. One of the primary benefits of IVIVC is that it allows for the assessment of how changes in in vitro dissolution can influence in vivo drug absorption, particularly when minor modifications are made to a formulation. Once a validated IVIVC model is developed, it can help predict bioavailability and bioequivalence (BA/BE) using existing in vitro data, potentially eliminating the need for clinical bioequivalence studies involving human participants.
Additionally, IVIVC enhances the understanding of the drug product itself, aiding in the establishment of broader acceptance criteria and improved formulation stability. It is particularly valuable for forecasting the in vivo implications of adjustments to formulation components, manufacturing locations, or processes. This is crucial during the initial stages of product development, but the benefits of IVIVC extend beyond that. After a product receives approval, an IVIVC model can be instrumental in assessing the effects of post-approval changes in manufacturing or issues related to specific production batches, all without necessitating costly in vivo bioequivalence studies.
Advantages of IVIVC
In vitro-in vivo correlation (IVIVC) offers numerous benefits across various submission types in drug development. It can be utilized to support:
- Abbreviated New Drug Applications (ANDA)
- New Drug Applications (NDA) for oral medications with extended release properties
- Abbreviated Antibiotic Drug Applications (AADA) as a substitute for in vivo bioequivalence (BE) assessments
Additionally, IVIVC can facilitate biowaivers, allowing sponsors to forgo certain in vivo bioavailability (BA) and BE studies when adequate safety and efficacy data are available. This is particularly useful when requesting biowaivers related to changes in drug manufacturing. Recently, IVIVC has been incorporated into the Quality by Design (QbD) framework, enabling the establishment of clinically relevant drug product specifications using dissolution as a key endpoint. IVIVC can help set dissolution specifications for:
- Justifying changes in strength
- Minor formulation adjustments
- Modifications in the manufacturing site
- Ensuring batch-to-batch quality control
FDA Guidelines for IVIVC
The FDA’s guidance document titled “Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations,” has been a key reference for over two decades, providing essential insights into regulatory expectations for IVIVC. When this guidance was published, accurately predicting the bioavailability characteristics of extended release products based on their dissolution profiles was a critical objective in the field. The document outlines:
- Steps for developing an IVIVC model
- Criteria for assessing predictability
- Methods for using IVIVC to set dissolution specifications
- Applications of IVIVC as a surrogate for in vivo BE studies

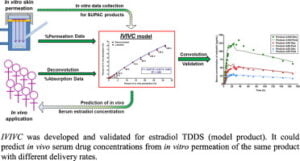
In-vitro-in vivo correlation
This guidance remains a cornerstone for understanding the regulatory landscape surrounding IVIVC for oral, extended release drug products.
Levels of IVIVC
IVIVC (In Vitro-In Vivo Correlation) is categorized into three main levels: A, B, and C, along with a subcategory known as multiple Level C correlation. Level A is the most widely used type, particularly in New Drug Applications (NDAs) and Investigational New Drug (IND) submissions. Level C serves as a valuable tool during early development phases and is the second most common, while Level B and multiple Level C correlations are less frequently encountered.
Level A
Level A correlation typically exhibits a linear relationship, illustrating a direct connection between in vitro dissolution rates and in vivo input rates; however, nonlinear relationships may also be applicable if suitable. This correlation is usually determined through a two-step process involving deconvolution, followed by a comparison of the absorbed drug quantity with the dissolved drug. Level A is recommended for demonstrating IVIVC among two or more formulations that have differing release rates. The FDA advocates for this level as it provides the most comprehensive insights. According to FDA guidelines, two or more formulations with varying release profiles (such as slow, medium, and fast releases differing by 10%) should be utilized to establish this relationship. For formulations where in vitro dissolution is unaffected by test conditions (e.g., medium, agitation, pH), a single formulation may suffice. The model should ideally forecast the complete in vivo time course based on in vitro data.
Level B
Level B correlation utilizes the same dataset as Level A but is grounded in statistical moment analysis. It compares the mean in vitro dissolution time of a drug with either the mean in vivo residence time or the mean in vivo dissolution time. This level is generally less effective for regulatory purposes, as it does not accurately represent the plasma level curves observed in vivo. Furthermore, in vitro data derived from a Level B correlation cannot adequately support quality control extremes.
Level C
Level C correlation focuses on the relationship between specific in vivo pharmacokinetic parameters (such as Cmax, AUC, and Ka) and in vitro dissolution data at a single time point. This type of correlation does not capture the entire shape of the plasma concentration-time curve, which is crucial for assessing the performance of extended-release products. It is also insufficient for justifying a bioequivalence waiver. However, Level C can predict Cmax and AUC, aiding in establishing bioavailability and bioequivalence.
Multiple Level C
Multiple Level C correlation extends the concept by relating one or more pharmacokinetic parameters to the amount of drug dissolved at various time points. This approach can be as informative as Level A correlation. However, if multiple Level C correlations can be established, it often suggests that Level A correlation is also probable and typically preferred. For studies in this category, sample sizes should range from 6 to 36 participants, using only human data. Crossover studies are preferred, but parallel studies may be acceptable. The reference product for developing an IVIVC may be intravenous, immediate-release, or an aqueous oral solution. While a fasting state is ideal, a fed state might be allowed for safety considerations.
General IVIVC Considerations
- Absorption Mechanism: A successful IVIVC relationship indicates that variations in the release rates of two or more formulations lead to corresponding differences in their absorption profiles, all through the same absorption mechanism.
- Release Rate Differences: For each formulation analyzed, the release rates (measured by the percentage dissolved) should vary by at least 10%. This variation should be reflected in the in vivo profiles, with notable differences in Cmax and AUC corresponding to the in vitro differences.
- Predictive Performance: The effectiveness of an IVIVC model is assessed through prediction error. This evaluation can utilize either internal datasets or external datasets, depending on the specific goals of the IVIVC analysis and the drug’s therapeutic area.
- Solubility Considerations: The likelihood of successfully developing an IVIVC is higher for drugs with good solubility, particularly when in vitro and in vivo data are available across various formulations, including a solution formulation as a reference.
- Challenges in Acceptance: Historically, the FDA has accepted less than 50% of IVIVC submissions. Common reasons for low success rates include:
- Inadequate selection of formulation types and amounts for IVIVC development and validation.
- Failure to analyze exploratory plots that can assist in model construction.
- Lack of investigation into factors that may lead to inconclusive predictability and the quality of input data.
- Insufficient selection of parameters for the model.

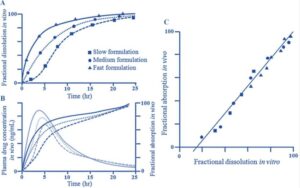
In-vitro-in vivo correlation
Conclusions
IVIVC offers numerous advantages, enhancing understanding of dosage forms and serving as a predictive tool to potentially reduce the need for certain clinical bioequivalence studies. It also aids in interpreting batch-to-batch variability and optimizing formulation development, thereby streamlining product development and manufacturing processes. However, creating an effective IVIVC model can be complex. If you’re interested in whether an IVIVC approach would suit your development program, reach out to Alucent. Our experienced consultants can help you develop robust IVIVC models tailored to your needs.